What we do
The right blend of different disciplines, technologies and competencies is often one of the factors to suceeding in our business and industry.
Our main engagements are projects related to therapeutical apheresis. One of cour core competencies in that area is the data management for pre-clinical studies and clinical studies.
Our location in Bitterfeld-Wolfen is dedicated to the synthesis of fine chemicals.
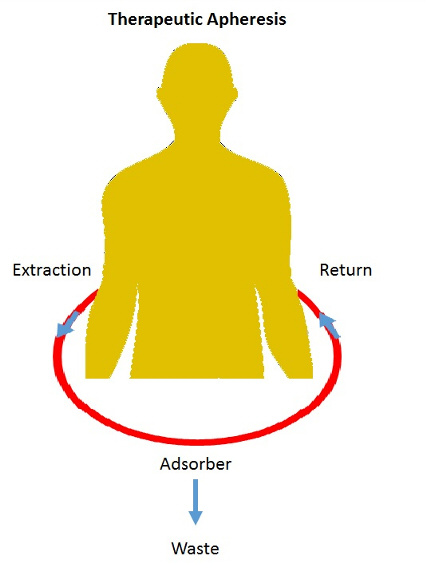
Many diseases are triggered by path molecules like autoantibodies or overexpression of molecules. The classical pharmacological strategy is to block the synthesis, e.g. enzymes or pathways.
The way from the target identification to a drug is very long and expensive. With the apheresis process the path molecules can be often removed by the absorber from the blood.
This can have a variety of advantages: - Our experience is that the development of a specific adsorber for the removal of molecules based on blood plasma or full blood requires less time than the classical strategies.
Once the adsorber is developed, the underlying medical hypothesis can be more easily tested since the application of the process is outside the corpus. This translates to an easier, faster, and cheaper certification process, since it is not the introduction of a new drug but the certification of a new medical device.
Our experience in certifying this process can be used as a template for your own certification. Dependent on your specific project, may be only the inner part of the adsorber, i.e the so called legand, which binds the unwanted molecules must be substituted, and therefore the re-use of our previous experience may promise an even faster and cheaper approach.
Furthermore, we have gained experience and acquired testing centers who have already gained expertise in applying apheresis in various scenarios.